The discovery of tantalum
Known as a “biophilic” metal, pure tantalum has been used in orthopedic medicine for more than 80 years. tantalum was discovered in ore by Swedish chemist Ekeberg in 1802 and named after Tantalus. tantalum was obtained in higher purity by the sodium reduction of Na2TaF7 by Rhodes in 1866.

Subsequently, with its unique physicochemical properties and excellent biological inertness and biocompatibility, pure tantalum was first used in the field of orthopedics in 1940 and has been clinically used for 80 years, becoming another new biomaterial after titanium, which is widely used in oral implant placement, femoral head necrosis treatment, coronary artery stent placement, artificial acetabular prosthesis implantation, surgical suture production and other medical-related fields. A large amount of literature confirms that no adverse reactions have occurred with pure tantalum as human implants.
Biological and physicochemical properties of tantalum
1) Physical properties of tantalum
Pure tantalum is a gray, bright, hard metal with moderate hardness and good ductility, and can be drawn into tantalum wires thinner than a hair.
2) Mechanical properties
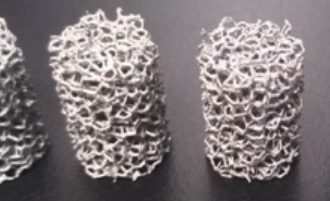
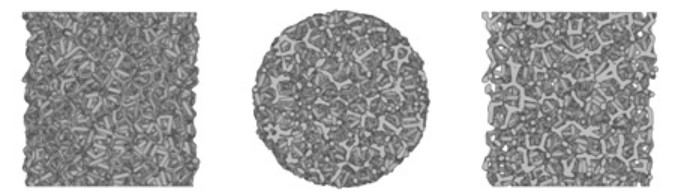
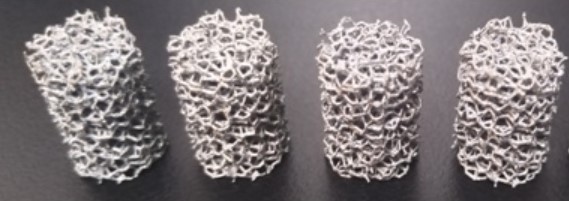
The difference between pure tantalum and the elastic modulus of bone tissue is large, which is not conducive to osseointegration. As a new type of orthopaedic implant material, porous tantalum has an interconnected internal structure and is interwoven longitudinally and horizontally. It has physical properties such as high porosity, low modulus of elasticity, and high surface friction coefficient.
In terms of mechanical properties, porous tantalum is superior to solid tantalum and other commonly used medical metal materials. Its special pore structure makes its elastic modulus between that of human cancellous bone and cortical bone, making it particularly suitable for bone replacement, joint replacement, and human tissue filling. It can provide mechanical strength while reducing stress shielding, facilitating stress transmission, facilitating bone shaping, and also has excellent osteoinductive properties, and is widely used in the field of orthopedics, and has achieved ideal results.
Compared with other porous materials, the friction coefficient of porous tantalum is 0.80 and 0.74 relative to the cancellous bone and cortical bone, respectively, which is 40% to 80% higher than that of traditional metallic materials. This helps to bond with the host bone interface and enhance the stability of the initial implantation.
3) Good corrosion resistance
At room temperature, tantalum will not react with hydrochloric acid, concentrated nitric acid or even aqua regia, and common inorganic salts will not corrode it. It is chemically extremely stable and resistant to all inorganic and organic acids except hydrofluoric acid, sulfur trioxide, hot concentrated sulfuric acid and alkalis.
4) Good biocompatibility
Unlike conventional medical metal materials, biological tissue grows on tantalum after a period of implantation, just as it does on real bone. This is why Tantalum is also known as a “pro-metal”.
5) Good bone bioactivity
The biologically active bone material interface is a hydroxyapatite layer rather than a connective tissue layer, and Tantalum itself has good bone bioactivity and stable biological inertness, which allows it to form a strong bone interface integration with bone.
Tantalum Applications
High clinical demand and large market size
The preparation of biocompatible bone scaffolds has been one of the hot topics of research in the medical field, and there are no less than 3 million cases of medical human bone implants in the USA every year, with prices reaching tens of thousands or even more than 100,000 yuan per case. According to EvaluateMedTech, orthopedic-related medical devices had global sales of $36.5 billion in 2017 and will reach $47.1 billion in 2024, with a compound annual growth rate of 3.7%.
Current orthopedic metal implant materials
The choice of medical human bone implant materials, the earlier application of materials are stainless steel, nickel-chromium alloy, nickel-titanium alloy, the last 2 or 3 years the trend is TC4 titanium alloy, these materials contain nickel, chromium, or aluminum, vanadium and other harmful elements, and because of its elastic modulus exceeds the human bone too much, the material and the human body affinity is low, prone to “bone non-stick “phenomenon. Medical experts and the market urgently need new non-toxic and non-hazardous new materials with good affinity to the human body to improve the current situation.
Porous tantalum advantages
(1) Perfect integration with the host bone interface: compared to the most commonly used titanium, tantalum is more biocompatible and has a better osseointegration capability.
(2) Unique bionic trabecular structure: Tantalum’s elastic modulus is closer to that of bone tissue, making it more suitable for bionic trabecular structure in the human body than other metals.
(3) Inducing rapid bone and vascular growth into It can promote the rapid growth of bone tissue and vascular tissue into the pores of porous tantalum, and its highly porous and supportive structure provides extensive space for bone growth, forming a good biological fixation, which can effectively solve the exothermic effect of bone cement and the impact on surrounding tissues, which is great clinical progress.
The above advantages make it show great clinical application value and application space in different sizes of orthopedic implants, and different parts of bone defects.
1) Application of porous tantalum in orthopedics
In clinical applications, porous tantalum printing can be applied to all small and medium-sized restorative products. For large-sized repair products, considering the high density of pure tantalum and the excessive weight of the printed implant prosthesis, multi-component gradient printing can be adopted, with porous tantalum used in the bone growing-in area and other metals such as titanium alloy, which is cheaper and lighter in quality, can be used in other areas.
With the continuous research on tantalum materials in recent years, several clinical trials have proven that new implants made of medical tantalum in combination with titanium and other metals can compensate for the shortcomings of other metal materials in terms of biocompatibility, bioactivity, and implant-bone bonding.
2) Tantalum coating – a new direction for orthopaedic applications
Tantalum metal has excellent corrosion resistance, and its coating on the surface of certain medical metal materials can effectively prevent the release of toxic elements and improve the biocompatibility of metal materials. Tantalum coatings meet the three elements of ideal bone graft materials, namely osteoconductivity, osteoconduction and osteogenesis, resulting in wider clinical applications and more flexible patient choices.
In addition, tantalum has also been used as an implant material in the restorative treatment of patients with missing teeth. Experiments have shown that conventional implants can absorb up to 30% of the loading energy during the loading process, while tantalum trabecular implants can absorb 50%-75%, which allows the implant to disperse the loading force to the surrounding bone during the long-term intraoral functional loading, avoiding stress concentration, while the higher friction coefficient provides good initial stability during implant placement, thus improving the dental implant bonding rate, especially in implant patients with poor bone quality.


















Recent Comments